JavaScript seems to be disabled in your browser. For the best experience on our site, be sure to turn on Javascript in your browser.
Click Here to Find How Many Items You Have Added:) 89030-95-5
Adding item(s) to cart......
Products are for research use only. Not for human use. We do not sell to patients.
Tel:(909) 407-4943 Email: sales@glpbio.com
Sample solution is provided at 25 µL, 10mM.
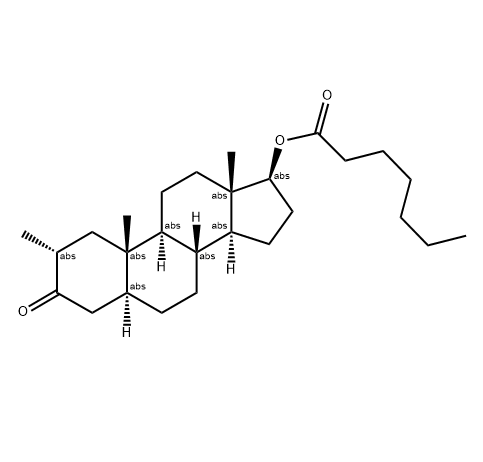
Testosterone undecanoate is an ester of testosterone; used in androgen replacement therapy primarily for the treatment of male hypogonadism, and is currently under research for use as a male contraceptive.
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section. )
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent. 2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving. 3. All of the above co-solvents are available for purchase on the GlpBio website.
Schering AG is developing a formulation of Testosterone undecanoate [Nebido] for the treatment of testosterone deficiency or hypogonadism. This deficiency can lead to decreased muscle mass, impaired muscle function, osteoporosis, reduced sexual function and mental degeneration. Schering claims that its new formulation provides superior control of blood levels of the drug and permits a longer period of application. Nebido requires only four injections per year, and represents a major improvement for men with testosterone deficiency. Schering AG received approval of its Testosterone undecanoate formulation in its first European country, Finland, in November 2003 for the treatment of hypogonadism in men. In July 2004, Schering's Testosterone undecanoate formulation completed approval of the European mutual recognition procedure. This approval clears the way for marketing the product (as Nebido) in the large pharmaceutical markets like Germany, France and the UK. The initial phase of the product launch will occur in Finland in October 2004, and in Germany in November 2004. Other European countries will follow in 2005, and following receipt of approval, it will be introduced in the first Latin American and Asian countries. In its 2002 Annual Report, Schering predicted that Testosterone undecanoate has the potential to reach peak sales of euro 100 million, 3 years after launch-launch in Europe was at the time anticipated in 2004.
Context: A novel formulation of oral testosterone (T) undecanoate (TU) was evaluated in a phase 3 clinical trial. Objective: Determine efficacy, short-term safety, and alignment of new oral TU formulation with current US approval standards for T replacement therapy. Design: Randomized, active-controlled, open-label study. Setting and patients: Academic and private clinical practice sites; enrolled patients were clinically hypogonadal men 18 to 65 years old. Methods: Patients were randomized 3:1 to oral TU, as prescribed (JATENZO®; n = 166) or a topical T product once daily (Axiron®; n = 56) for 3 to 4 months. Dose titration was based on average T levels (Cavg) calculated from serial pharmacokinetic (PK) samples. T was assayed by liquid chromatography-mass spectrometry/mass spectrometry. Patients had 2 dose adjustment opportunities prior to final PK visit. Safety was assessed by standard clinical measures, including ambulatory blood pressure (BP). Results: 87% of patients in both groups achieved mean T Cavg in the eugonadal range. Sodium fluoride-ethylenediamine tetra-acetate plasma T Cavg (mean ± standard deviation) for the oral TU group was 403 ± 128 ng/dL (~14 ± 4 nmol/L); serum T equivalent, ~489 ± 155 ng/dL (17 ± 5 nmol/L); and topical T, 391 ± 140 ng/dL (~14 ± 5 nmol/L). Modeling/simulation of T PK data demonstrated that dose titration based on a single blood sample 4 to 6 h after oral TU dose yielded efficacy (93%) equivalent to Cavg-based titration (87%). Safety profiles were similar in both groups, but oral TU was associated with a mean increase in systolic BP of 3 to 5 mm Hg. Conclusion: A new oral TU formulation effectively restored T to mid-eugonadal levels in hypogonadal patients.
Importance of the field: Testosterone undecanoate (TU) represents an exciting new testosterone replacement therapy for hypogonadal men due to its convenient dosing schedule and favorable pharmacokinetic and safety profiles. Areas covered in this review: Clinical, pharmacokinetic and safety characteristics of TU will be reviewed. The characteristics of currently approved testosterone therapies will be reviewed and compared with those of TU in order to determine which therapy most appropriately meets the clinical objective of properly matching a patient with a therapy that is best able to deliver physiological levels of testosterone for prolonged periods of time, while at the same time being safe, effective, inexpensive, simple to use, and with few side effects. What the reader will gain: TU represents the first long-acting injectable with an excellent safety profile that can be administered only four times annually to produce stable levels of testosterone. Long-term studies have validated the clinical efficacy of TU in maintaining therapeutic levels of testosterone. Patient preference for the convenient dosing schedule might also lead to better compliance and therapeutic benefit. No serious side effects have been noted with the use of TU, including long-term data on patients treated with TU over 8 years. Take home message: TU is both a desirable and safe option for the treatment of hypogonadal men. Patients will benefit from the stable testosterone levels and fewer required injections, while achieving the desired benefits of androgen replacement.
Introduction: Injectable Testosterone undecanoate (TU) is a long-acting testosterone (T) formulation available for the treatment of male hypogonadism (HG) since 2003. Areas covered: The efficacy and safety of injectable TU are assessed, as obtained by meta-analyzing available evidence. An extensive Medline, Embase and Cochrane search was performed. All uncontrolled and placebo-controlled randomized clinical trials (RCTs), evaluating the effect of injectable TU on different outcomes, were included. Of the 98 retrieved articles, 33 were included in the study. Among those, 11 were placebo-controlled RCTs. Injectable TU was significantly associated with a reduction of fat mass and HbA1c in both controlled and uncontrolled trials, in particular when hypogonadal subjects were enrolled. Similar results were observed for the improvement of erectile function. In addition, TU ameliorated several other outcomes, including blood pressure, lipid profile, waist circumference and body mass index in uncontrolled studies, but these data were not confirmed in placebo-controlled trials. The treatment was well tolerated and no risk of prostate cancer or cardiovascular disease was observed. Expert opinion: Injectable TU is a safe and effective treatment for male HG. The possibility of a therapeutic intervention just four to five times per year frees the patient, at least partially, from having a chronic condition, thus maintaining a positive, active role in self-caring.
Testosterone undecanoate injections (TU), an oil-based depot, is a universal hormonal-based treatment which has been associated with pulmonary oil microembolism (POME). However, the rate of POME during routine intramuscular (IM) TU injection is unknown. Here, we conduct a peer-reviewed literature review investigating POME incidents in the setting of TU injections. A total of 48 articles were selected in the literature review, which included 29 studies that used TU and reported its effects. Relatively few POME cases were reported across multiple published studies, including those that focused particularly on the occurrence rate of POME while administrating IM TU. Of the 29 individual studies, which included 7 978 patients, eight studies reported a total of 88 incidence of POME cases or cough. This included episodes of cough that were not originally declared as POME. One post market review reported 223 cases per 3,107,652 injections. When POME did occur, almost all cases resolved spontaneously within 60 min without intervention. Overall, POME was observed to be rare.
Average Rating: 5 ★★★★★ (Based on Reviews and 7 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY . Please make sure your review or question is research based. Required fields are marked with *
You may receive emails regarding this submission. Any emails will include the ability to opt-out of future communications.
Research Chemicals Your information is safe with us.Privacy Policy